
Chemistry, 09.06.2021 09:50 aliceohern
A solution containing 1.22 g of a diprotic acid H2CH2O4 (malonic acid)
was titrated with 45.5 mL of NaOH to reach the second equivalence
point. What is the concentration of the NaOH solution? (MW_malonic
acid = 104.06 g/mol)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1
You know the right answer?
A solution containing 1.22 g of a diprotic acid H2CH2O4 (malonic acid)
was titrated with 45.5 mL of...
Questions


Business, 30.09.2021 19:40

Geography, 30.09.2021 19:40

Geography, 30.09.2021 19:40



Mathematics, 30.09.2021 19:40



Biology, 30.09.2021 19:40

Social Studies, 30.09.2021 19:40

Mathematics, 30.09.2021 19:40


Mathematics, 30.09.2021 19:40

Mathematics, 30.09.2021 19:40


Business, 30.09.2021 19:40



Business, 30.09.2021 19:40

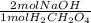 = 0.02344 mol NaOH
= 0.02344 mol NaOH

