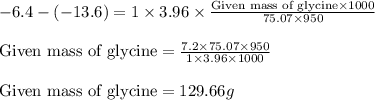Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
A certain liquid has a normal freezing point of and a freezing point depression constant . A solutio...
Questions


Geography, 08.12.2020 19:10




Mathematics, 08.12.2020 19:10

Mathematics, 08.12.2020 19:10

History, 08.12.2020 19:10




Mathematics, 08.12.2020 19:10

Mathematics, 08.12.2020 19:10


Health, 08.12.2020 19:10

Mathematics, 08.12.2020 19:10



Mathematics, 08.12.2020 19:10

Geography, 08.12.2020 19:10

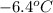 and a molal freezing point depression constant
and a molal freezing point depression constant 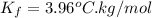 . A solution is prepared by dissolving some glycine in 950. g of X. This solution freezes at
. A solution is prepared by dissolving some glycine in 950. g of X. This solution freezes at 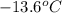 . Calculate the mass of urea that was dissolved. Round your answer to 2 significant digits.
. Calculate the mass of urea that was dissolved. Round your answer to 2 significant digits.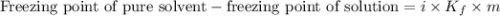
 ....(1)
....(1)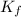 = freezing point depression constant =
= freezing point depression constant = 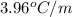
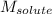 = Molar mass of solute (glycine) = 75.07 g/mol
= Molar mass of solute (glycine) = 75.07 g/mol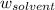 = Mass of solvent = 950 g
= Mass of solvent = 950 g