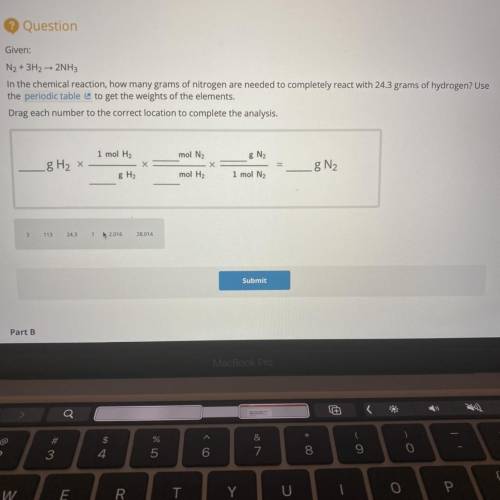
Given:
N2 + 3H2 → 2NH3
In the chemical reaction, how many grams of nitrogen are needed to com...

Chemistry, 09.06.2021 01:40 nadiarose6366
Given:
N2 + 3H2 → 2NH3
In the chemical reaction, how many grams of nitrogen are needed to completely react with 24.3 grams of hydrogen? Use
the periodic table to get the weights of the elements.
Drag each number to the correct location to complete the analysis.
1 mol H2
mol N2
8 N2
8 H2 x
g N2
8 H2
mol H2
1 mol N2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

You know the right answer?
Questions




History, 22.09.2019 07:10




Physics, 22.09.2019 07:10



History, 22.09.2019 07:10



Biology, 22.09.2019 07:10


Chemistry, 22.09.2019 07:10

Mathematics, 22.09.2019 07:10

Mathematics, 22.09.2019 07:10

Mathematics, 22.09.2019 07:10



