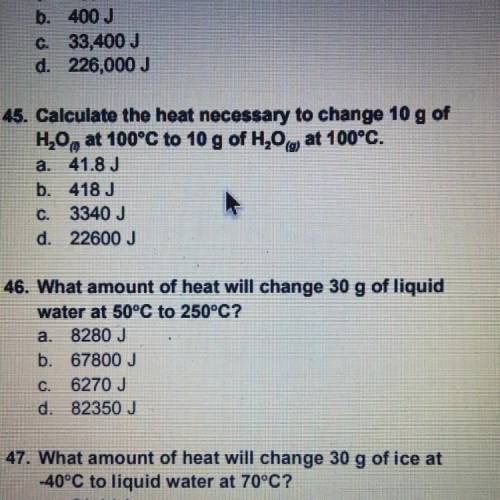Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1
You know the right answer?
Calculate the heat necessary to change 10 g of H20, at 100°C to 10 g of H20 at 100°C.
a. 41.8 J
Questions


Mathematics, 26.11.2020 14:00

Law, 26.11.2020 14:00

English, 26.11.2020 14:00

Social Studies, 26.11.2020 14:00

Social Studies, 26.11.2020 14:00

Biology, 26.11.2020 14:00



Chemistry, 26.11.2020 14:00

Biology, 26.11.2020 14:00

Mathematics, 26.11.2020 14:00



English, 26.11.2020 14:00



Mathematics, 26.11.2020 14:00

English, 26.11.2020 14:00

Chemistry, 26.11.2020 14:00