
Chemistry, 08.06.2021 16:40 AbigailHaylei
4Fe (s) + 3 O2 (g) → 2 Fe2O3 (s)
A. What is the oxidation number for each item in this equation?
B. What is being oxidized?
C. What is being reduced?
D. What is the oxidizing agent?
E. What is the reducing agent?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 11:20
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2
You know the right answer?
4Fe (s) + 3 O2 (g) → 2 Fe2O3 (s)
A. What is the oxidation number for each item in this equation?
Questions





Mathematics, 10.03.2021 04:50

Geography, 10.03.2021 04:50

Biology, 10.03.2021 04:50


Mathematics, 10.03.2021 04:50

Chemistry, 10.03.2021 04:50

Mathematics, 10.03.2021 04:50

Mathematics, 10.03.2021 04:50

Mathematics, 10.03.2021 04:50







History, 10.03.2021 04:50

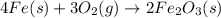
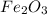 is +3.
is +3. 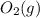 is 0 as it is present in its elemental state.
is 0 as it is present in its elemental state.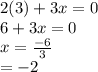
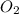 as a decrease in its oxidation state is occurring. So,
as a decrease in its oxidation state is occurring. So, 

