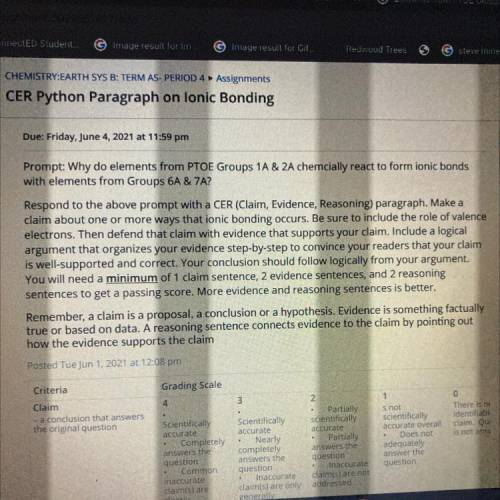
CER Python Paragraph on lonic Bonding
Due: Friday, June 4, 2021 at 11:59 pm
Prompt: Why do el...

Chemistry, 08.06.2021 09:10 jackbruski
CER Python Paragraph on lonic Bonding
Due: Friday, June 4, 2021 at 11:59 pm
Prompt: Why do elements from PTOE Groups 1A & 2A chemcially react to form ionic bonds
with elements from Groups 6A & 7A?
Respond to the above prompt with a CER (Claim, Evidence, Reasoning) paragraph. Make a
claim about one or more ways that ionic bonding occurs. Be sure to include the role of valence
electrons. Then defend that claim with evidence that supports your claim. Include a logical
argument that organizes your evidence step-by-step to convince your readers that your claim
is well-supported and correct. Your conclusion should follow logically from your argument.
You will need a minimum of 1 claim sentence, 2 evidence sentences, and 2 reasoning
sentences to get a passing score. More evidence and reasoning sentences is better.
Remember, a claim is a proposal, a conclusion or a hypothesis. Evidence is something factually
true or based on data. A reasoning sentence connects evidence to the claim by pointing out
how the evidence supports the claim

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Questions

Mathematics, 20.01.2021 05:50

Biology, 20.01.2021 05:50

Biology, 20.01.2021 05:50

Geography, 20.01.2021 05:50

History, 20.01.2021 05:50

Mathematics, 20.01.2021 05:50


Biology, 20.01.2021 05:50



World Languages, 20.01.2021 05:50

English, 20.01.2021 05:50

Engineering, 20.01.2021 05:50

Mathematics, 20.01.2021 05:50

Biology, 20.01.2021 05:50

Mathematics, 20.01.2021 05:50

Mathematics, 20.01.2021 05:50

Social Studies, 20.01.2021 05:50

Mathematics, 20.01.2021 05:50



