
Chemistry, 07.06.2021 22:50 naomicervero
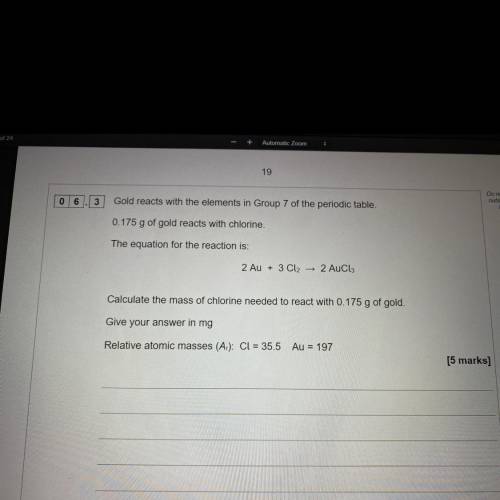
Gold reacts with elements in group 7 of the periodic table 0.175g of gold reacts with chlorine.
The equation for reaction for the reaction is
2Au + 3cl2 ——> 2Aucl3
Calculate the mass of chlorine needed to react with 0.175g of gold. Give your answer in mg
Relative atomic masses cl=35.5 Au=197

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Gold reacts with elements in group 7 of the periodic table 0.175g of gold reacts with chlorine.
The...
Questions

Mathematics, 28.01.2020 00:31


Mathematics, 28.01.2020 00:31

Mathematics, 28.01.2020 00:31

Biology, 28.01.2020 00:31




History, 28.01.2020 00:31

Mathematics, 28.01.2020 00:31

Physics, 28.01.2020 00:31



English, 28.01.2020 00:31




English, 28.01.2020 00:31

History, 28.01.2020 00:31

Mathematics, 28.01.2020 00:31



