
Chemistry, 07.06.2021 01:00 kell22wolf
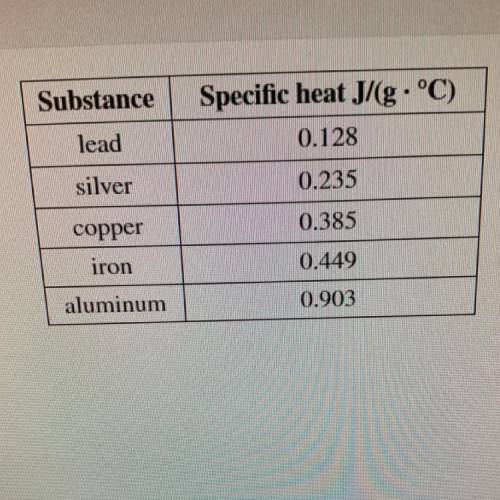
The temperature of a sample of silver increased by 23.8 °C
when 261 J of heat was applied.
What is the mass of the sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
The temperature of a sample of silver increased by 23.8 °C
when 261 J of heat was applied.
Wh...
Wh...
Questions


Mathematics, 02.03.2021 19:40


History, 02.03.2021 19:40




Mathematics, 02.03.2021 19:40


English, 02.03.2021 19:40


Chemistry, 02.03.2021 19:40

Mathematics, 02.03.2021 19:40





Mathematics, 02.03.2021 19:40


History, 02.03.2021 19:40



