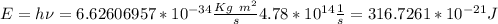Chemistry, 06.06.2021 19:30 denoterap441wb
Calculate the energy of the orange light emitted, per photon, by a neon sign with a frequency of 4.78 × 1014 Hz.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Calculate the energy of the orange light emitted, per photon, by a neon sign with a frequency of 4.7...
Questions

Mathematics, 14.11.2020 02:20


Biology, 14.11.2020 02:20

Mathematics, 14.11.2020 02:20




Mathematics, 14.11.2020 02:20


Mathematics, 14.11.2020 02:20

History, 14.11.2020 02:20





Health, 14.11.2020 02:20

Mathematics, 14.11.2020 02:20


Mathematics, 14.11.2020 02:20

English, 14.11.2020 02:20