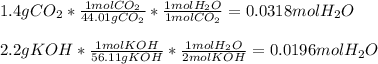Chemistry, 06.06.2021 01:10 Kaylenejohnson00
Identify the limiting reactant
1.4 g of CO2 and 2.2 g of KOH in the reaction: CO2 + 2KOH → K2CO3 + H20
Please show work, will give brainliest

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
Answers: 1

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

You know the right answer?
Identify the limiting reactant
1.4 g of CO2 and 2.2 g of KOH in the reaction: CO2 + 2KOH → K2CO3 +...
Questions

History, 24.12.2019 01:31

Mathematics, 24.12.2019 01:31

English, 24.12.2019 01:31

Social Studies, 24.12.2019 01:31

Spanish, 24.12.2019 01:31

Mathematics, 24.12.2019 01:31

Mathematics, 24.12.2019 01:31

Biology, 24.12.2019 01:31

Social Studies, 24.12.2019 01:31

Biology, 24.12.2019 01:31

Mathematics, 24.12.2019 01:31

Advanced Placement (AP), 24.12.2019 01:31


Mathematics, 24.12.2019 01:31

Health, 24.12.2019 01:31

History, 24.12.2019 01:31

Mathematics, 24.12.2019 01:31