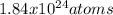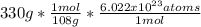Calculate number of silver atoms in
pure,
silver bracelet that
mass of 330, CAg=108. Na =...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
Questions


Health, 04.03.2021 07:40


Mathematics, 04.03.2021 07:40

Mathematics, 04.03.2021 07:40

Mathematics, 04.03.2021 07:40


Mathematics, 04.03.2021 07:40

Mathematics, 04.03.2021 07:40


Mathematics, 04.03.2021 07:40

Mathematics, 04.03.2021 07:50

Biology, 04.03.2021 07:50

English, 04.03.2021 07:50

Physics, 04.03.2021 07:50

Social Studies, 04.03.2021 07:50

Mathematics, 04.03.2021 07:50

Mathematics, 04.03.2021 07:50

Mathematics, 04.03.2021 07:50

Biology, 04.03.2021 07:50