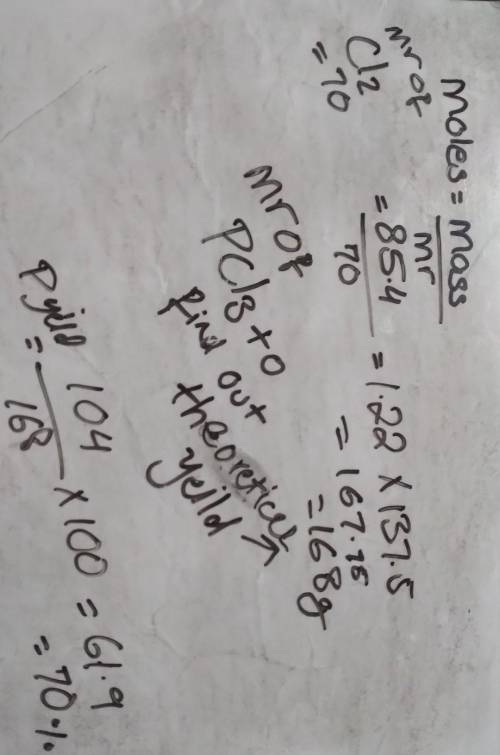Chemistry, 05.06.2021 22:40 Angeldelissa
85.4 g of chlorine reacts completely according to the following reaction: P4 (s) + 6 Cl2, (g) 4PC13 (I)
If 104 g of phosphorous trichloride is produced, what is the percent yield for this reaction?
please show work
will give brainliest

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
85.4 g of chlorine reacts completely according to the following reaction: P4 (s) + 6 Cl2, (g) 4PC13...
Questions


English, 12.07.2019 14:30




Health, 12.07.2019 14:30


Mathematics, 12.07.2019 14:30


Chemistry, 12.07.2019 14:30

Social Studies, 12.07.2019 14:30



Social Studies, 12.07.2019 14:30

Mathematics, 12.07.2019 14:30



Social Studies, 12.07.2019 14:30

Mathematics, 12.07.2019 14:30