
Chemistry, 05.06.2021 08:00 skatingflower
What is the balanced equation for the
equilibrium reaction where the aqueous
bicarbonate ion, HCO3(aq); decomposes to
form hydrogen ions, H,
(aq), and carbonate
ions, CO2 ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

You know the right answer?
What is the balanced equation for the
equilibrium reaction where the aqueous
bicarbonate ion,...
bicarbonate ion,...
Questions

Spanish, 24.07.2019 16:00

History, 24.07.2019 16:00

History, 24.07.2019 16:00



Biology, 24.07.2019 16:00

Computers and Technology, 24.07.2019 16:00



Mathematics, 24.07.2019 16:00



English, 24.07.2019 16:00


Social Studies, 24.07.2019 16:00




English, 24.07.2019 16:00

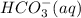 ; decomposes to form hydrogen ions,
; decomposes to form hydrogen ions, 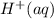 and carbonate ions,
and carbonate ions, 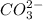 is
is 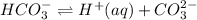 .
.

