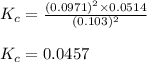Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3
You know the right answer?
At a certain temperature, 0.700 mol SO3 is placed in a 3.50 L container. 2SO3(g)↽−−⇀2SO2(g)+O2(g) At...
Questions


Mathematics, 27.02.2020 08:22



Mathematics, 27.02.2020 08:23

Mathematics, 27.02.2020 08:23

Mathematics, 27.02.2020 08:23

Biology, 27.02.2020 08:24

Mathematics, 27.02.2020 08:24


English, 27.02.2020 08:25

Mathematics, 27.02.2020 08:25

Mathematics, 27.02.2020 08:25





History, 27.02.2020 08:37

Mathematics, 27.02.2020 08:37

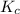 for the given chemical equation is 0.0457.
for the given chemical equation is 0.0457.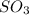 = 0.700 moles
= 0.700 moles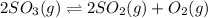
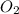 = x = 0.180 moles
= x = 0.180 moles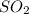 = 2x =
= 2x = 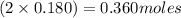
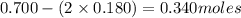
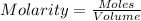
![[SO_3]_{eq}=\frac{0.340}{3.50}=0.0971M](/tpl/images/1363/8127/df11a.png)
![[SO_2]_{eq}=\frac{0.360}{3.50}=0.103M](/tpl/images/1363/8127/460c1.png)
![[O_2]_{eq}=\frac{0.180}{3.50}=0.0514M](/tpl/images/1363/8127/c4822.png)
![K_c=\frac{[SO_2]^2[O_2]}{[SO_3]^2}](/tpl/images/1363/8127/ea3f9.png)