
Chemistry, 04.06.2021 23:20 alex12everett
A monoprotic weak acid when dissolved in water is 0.66% dissociated and produces a solution with a pH of 3.04. Calculate the Ka of the acid. g

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
A monoprotic weak acid when dissolved in water is 0.66% dissociated and produces a solution with a p...
Questions



History, 25.06.2019 13:40


English, 25.06.2019 13:40

Chemistry, 25.06.2019 13:40

Mathematics, 25.06.2019 13:40

Mathematics, 25.06.2019 13:40


Chemistry, 25.06.2019 13:40

English, 25.06.2019 13:40







English, 25.06.2019 13:40


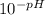 [H⁺] = 9.12x10⁻⁴ M
[H⁺] = 9.12x10⁻⁴ M![\frac{[9.12x10^{-4}]*[9.12x10^{-4}]}{[0.138]}](/tpl/images/1363/4628/7f320.png) = 6.02x10⁻⁶
= 6.02x10⁻⁶

