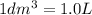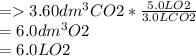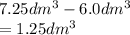Chemistry, 04.06.2021 20:20 juliannabartra
Propane burns to form carbon dioxide and water. The equation for the reaction is:
C 3 H 8 (g)+5 O 2 (g) 3 CO 2 (g)+4 H 2 O(l)
3.60d * m ^ 3 carbon dioxide is produced when a sample of propane is burned in 7.25d * m ^ 3 oxygen.
Calculate the volume of unreacted oxygen. Give your answer in cm^ 3

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1
You know the right answer?
Propane burns to form carbon dioxide and water. The equation for the reaction is:
C 3 H 8 (g)+5 O 2...
Questions

Mathematics, 15.04.2021 17:00




Physics, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00







English, 15.04.2021 17:00


Biology, 15.04.2021 17:00


Mathematics, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00

Mathematics, 15.04.2021 17:00