Pb(NO3)2 + AlBr3 →


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1
You know the right answer?
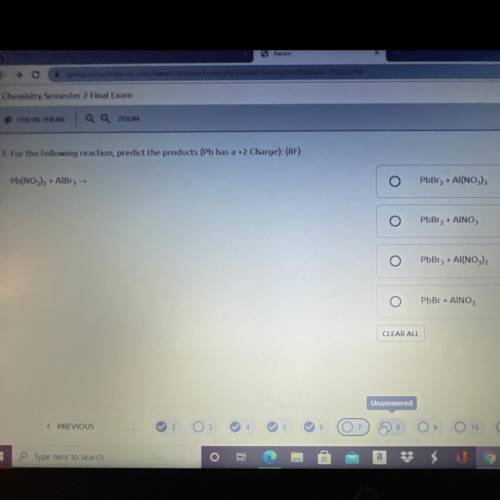
For the following reaction, predict the products (Pb has a +2 Charge):
Pb(NO3)2 + AlBr3 →
Pb(NO3)2 + AlBr3 →
Questions

Mathematics, 21.11.2019 10:31

Physics, 21.11.2019 10:31



Mathematics, 21.11.2019 10:31


Mathematics, 21.11.2019 10:31


Mathematics, 21.11.2019 10:31

Mathematics, 21.11.2019 10:31




English, 21.11.2019 10:31

Mathematics, 21.11.2019 10:31


Chemistry, 21.11.2019 10:31