Chemistry, 04.06.2021 04:30 krazyapril4601
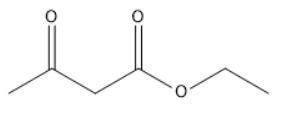
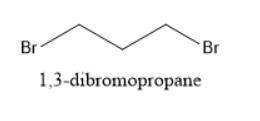
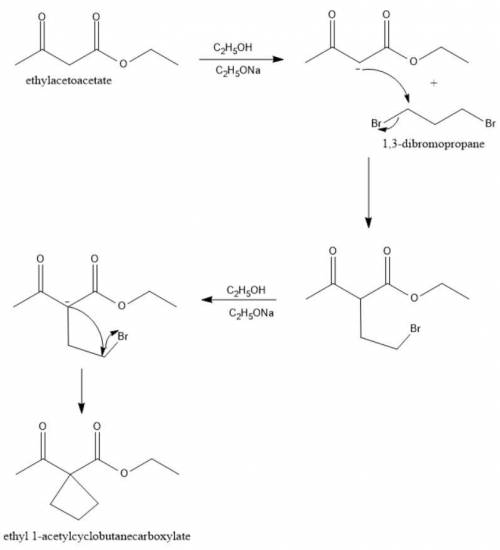
When Ethyl Acetoacetate is treated with 1,3-Dibromopropane and 2 moles of Sodium Ethoxide in Ethanol, a compound is produced that has the formula C9H14O3. This compound has an infrared spectrum that shows only one carbonyl adsorption and no OH bond stretch. Suggest a structure for this compound, and provide a mechanism for its formation.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1


Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
When Ethyl Acetoacetate is treated with 1,3-Dibromopropane and 2 moles of Sodium Ethoxide in Ethanol...
Questions

Mathematics, 25.03.2021 02:30

Mathematics, 25.03.2021 02:30

Social Studies, 25.03.2021 02:30



Social Studies, 25.03.2021 02:30




History, 25.03.2021 02:30

Mathematics, 25.03.2021 02:30




Mathematics, 25.03.2021 02:30

Mathematics, 25.03.2021 02:30


Mathematics, 25.03.2021 02:30

Mathematics, 25.03.2021 02:30