
Chemistry, 04.06.2021 03:50 toribrown3773
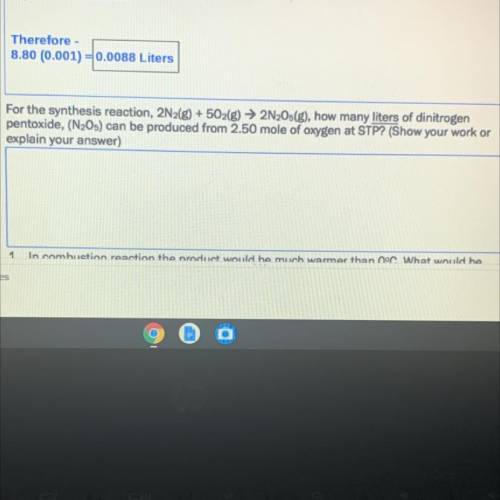
For the synthesis reaction, 2N2(g) + 5O2(g) → 2N2Os(g), how many liters of dinitrogen
pentoxide, (N205) can be produced from 2.50 mole of oxygen at STP? (Show your work or
explain your answer)
*SEE PICTURE FOR BETTER UNDERSTANDING*

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
For the synthesis reaction, 2N2(g) + 5O2(g) → 2N2Os(g), how many liters of dinitrogen
pentoxide, (N...
Questions



Mathematics, 05.11.2020 06:50

Arts, 05.11.2020 06:50








Mathematics, 05.11.2020 06:50


English, 05.11.2020 06:50

Mathematics, 05.11.2020 06:50


Mathematics, 05.11.2020 06:50


Mathematics, 05.11.2020 06:50



