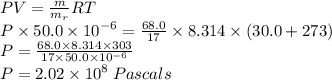Chemistry, 03.06.2021 17:50 justintisdale95
What is the pressure exerted by 68.0 g of nitrogen trihydride gas in a 50.0L container at 30.0 C?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
What is the pressure exerted by 68.0 g of nitrogen trihydride gas in a 50.0L container at 30.0 C?...
Questions


Mathematics, 04.02.2021 09:50

Mathematics, 04.02.2021 09:50

English, 04.02.2021 09:50



History, 04.02.2021 09:50

History, 04.02.2021 09:50



Chemistry, 04.02.2021 09:50

Mathematics, 04.02.2021 09:50


Biology, 04.02.2021 09:50

Mathematics, 04.02.2021 09:50

Mathematics, 04.02.2021 09:50