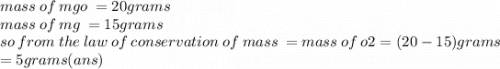Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1


Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

You know the right answer?
I will love u forever if u help me + branliest
Use the following equation: 2Mg + O2 → 2MgO
Questions

English, 08.04.2021 16:00

Chemistry, 08.04.2021 16:00




Mathematics, 08.04.2021 16:00





Physics, 08.04.2021 16:00









English, 08.04.2021 16:00