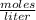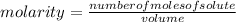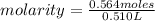Chemistry, 03.06.2021 07:20 yselahernandez02
a chemist dissolves 0.564 moles of manganese (IV) oxide (MnO2) in water, and adds enough water to make 0.510 L of solution. Calculate the molarity of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
a chemist dissolves 0.564 moles of manganese (IV) oxide (MnO2) in water, and adds enough water to ma...
Questions




Mathematics, 18.03.2021 03:20

Arts, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

History, 18.03.2021 03:20



Business, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20



Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20