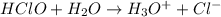Write the Ka expression for an aqueous solution of hypochlorous acid: (Note that either the numerator or denominator may contain more than one chemical species. Enter the complete numerator in the top box and the complete denominator in the bottom box. Remember to write the hydronium ion out as , and not as )

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Write the Ka expression for an aqueous solution of hypochlorous acid: (Note that either the numerato...
Questions

Social Studies, 19.07.2019 23:30

History, 19.07.2019 23:30


English, 19.07.2019 23:30

History, 19.07.2019 23:30

Biology, 19.07.2019 23:30

Business, 19.07.2019 23:30


Health, 19.07.2019 23:30

Social Studies, 19.07.2019 23:30

Mathematics, 19.07.2019 23:30


Health, 19.07.2019 23:30


Biology, 19.07.2019 23:30

Social Studies, 19.07.2019 23:30


Mathematics, 19.07.2019 23:30


Geography, 19.07.2019 23:30

![K_{a} = \frac{[H_{3}O^{+}][OCl^{-}]}{[HClO]}](/tpl/images/1360/4072/7fa94.png) .
.