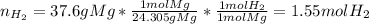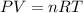Chemistry, 03.06.2021 01:00 piratesfc02
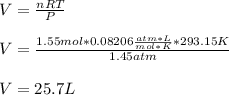
What volume of hydrogen will produced at 1.45 atm and a tempeture of 20C by the reaction of 37.6g of magnesium 1Mg+2H2O--> Mg(OH)2+H2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2


Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
What volume of hydrogen will produced at 1.45 atm and a tempeture of 20C by the reaction of 37.6g of...
Questions




Computers and Technology, 12.07.2019 17:10


History, 12.07.2019 17:10

Mathematics, 12.07.2019 17:10



Mathematics, 12.07.2019 17:10


History, 12.07.2019 17:10


English, 12.07.2019 17:10

Mathematics, 12.07.2019 17:10

Computers and Technology, 12.07.2019 17:10

Mathematics, 12.07.2019 17:10



Computers and Technology, 12.07.2019 17:10