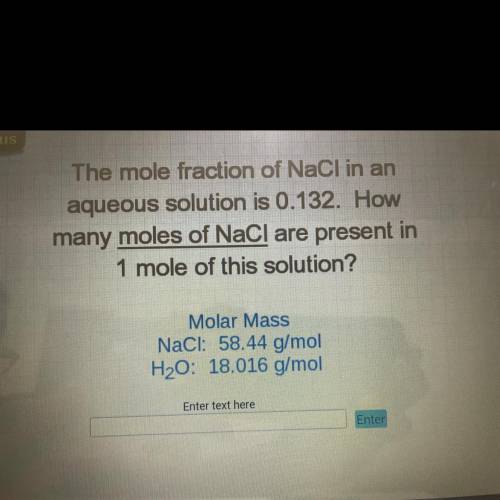
The mole fraction of NaCl in an
aqueous solution is 0.132. How
many moles of NaCl are present...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
Questions

History, 10.12.2020 01:00

Computers and Technology, 10.12.2020 01:00



Mathematics, 10.12.2020 01:00

Physics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Physics, 10.12.2020 01:00




Mathematics, 10.12.2020 01:00


Mathematics, 10.12.2020 01:00

History, 10.12.2020 01:00

English, 10.12.2020 01:00

Engineering, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Computers and Technology, 10.12.2020 01:00