
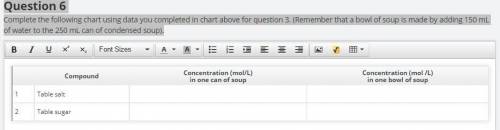
Question 6
Complete the following chart using data you completed in chart above for question 3. (Remember that a bowl of soup is made by adding 150 mL of water to the 250 mL can of condensed soup).
Question 7
Imagine one batch of soup (Batch “A”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (Batch “B”) is made with 8.32 g/can of salt.
Part A
Explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Part B
Explain which batch would have the higher boiling point and explain why.
Part C
If you want to have better frost protection, you can add an excess of 0.0200 moles of salt or 0.0200 moles of sugar. Explain which would result in the greater frost protection – sugar or salt. Justify your response.
Part D
How does Raoult’s law enable you to decide which batch of soup would have the greater vapor pressure at the same temperature? Describe this in 2-3 sentences.
Question 8
Do an online search investigating careers in food chemistry. Write a 250 word article commenting on suitable characteristics for this career, potential pathways of training, typical workplace tasks, opportunities for advancement, potential employers and potential income.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
Question 6
Complete the following chart using data you completed in chart above for question 3. (Re...
Questions









History, 16.11.2019 01:31

Physics, 16.11.2019 01:31

Social Studies, 16.11.2019 01:31

History, 16.11.2019 01:31



Social Studies, 16.11.2019 01:31



Mathematics, 16.11.2019 01:31

English, 16.11.2019 01:31



