

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1


Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
200 grams of iron (III) chloride reacts with ammonium carbonate [(NH4)2CO3] in the following equatio...
Questions

Mathematics, 07.05.2021 21:30

Social Studies, 07.05.2021 21:30







Mathematics, 07.05.2021 21:30

Mathematics, 07.05.2021 21:30


Mathematics, 07.05.2021 21:30

History, 07.05.2021 21:30


Mathematics, 07.05.2021 21:30



Biology, 07.05.2021 21:30

Geography, 07.05.2021 21:30

Mathematics, 07.05.2021 21:30

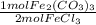 = 0.616 mol Fe₂(CO₃)₃
= 0.616 mol Fe₂(CO₃)₃

