
Chemistry, 02.06.2021 05:40 lillyawesome28
Silver nitrate, AgNO3, reacts with ferric chloride, FeCl3, to give silver chloride, AgCl, and ferric nitrate, Fe(NO3)3. In a particular experiment, it was planned to mix a solution containing 25.0 g of AgNO3 with another solution containing 45.0 grams of FeCl3. Write the chemical equation for the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3
You know the right answer?
Silver nitrate, AgNO3, reacts with ferric chloride, FeCl3, to give silver chloride, AgCl, and ferric...
Questions


Mathematics, 06.11.2020 21:10

Biology, 06.11.2020 21:10





Law, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10



Mathematics, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Biology, 06.11.2020 21:10


Mathematics, 06.11.2020 21:10


English, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

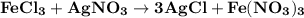
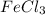 = 45 g
= 45 g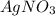 = 25g
= 25g

