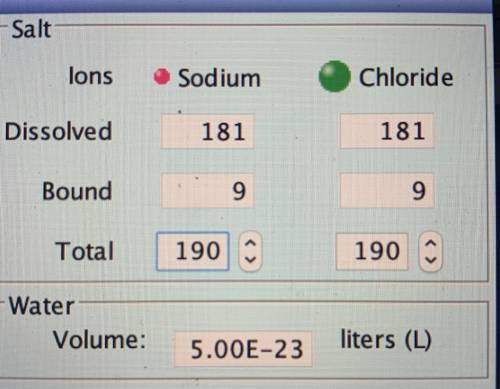
The solubility of an ionic compound can be expressed as the number of moles of the compound that will dissolve per liter of solution (molarity). The saturated solution has approximately(a) sodium ions dissolved in it (give an estimate of the average value.) The solution (not the solid) contains approximately(b) moles of sodium ions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
The solubility of an ionic compound can be expressed as the number of moles of the compound that wil...
Questions


Mathematics, 03.07.2019 20:30





Mathematics, 03.07.2019 20:30

Mathematics, 03.07.2019 20:30







Mathematics, 03.07.2019 20:30




Mathematics, 03.07.2019 20:30