
An investigation is conducted into how the mass of magnesium metal reacting with hydrochloric acid affects the amount of hydrogen gas produced.
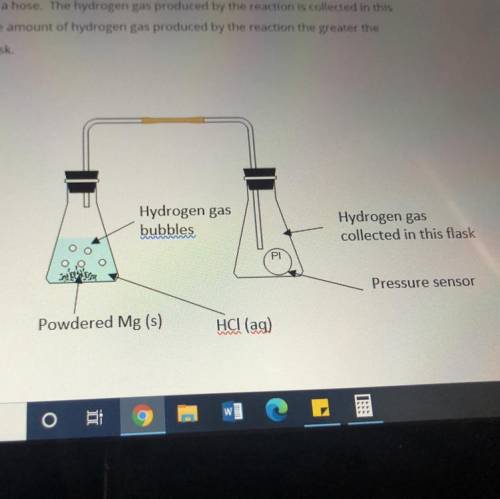
Masses of 0.10g, 0.20g, 0.30g and 0.40g of powdered Mg metal are reacted with hydrochloric acid(HCl). The conical flask containing the reaction mixture of Mg and HCl is connected to another conical flask with a hose. The hydrogen gas produced by the reaction is collected in this conical flask. The greater the amount of hydrogen gas produced by the reaction the greater the pressure of the gas in the flask.
A) what is the independent variable:
B) what is the dependent variable:
C) write a hypothesis for this investigation:
D) give 2 variables that should have been controlled for this investigation:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

You know the right answer?
An investigation is conducted into how the mass of magnesium metal reacting with hydrochloric acid a...
Questions


Mathematics, 04.02.2021 04:10


Mathematics, 04.02.2021 04:10



Mathematics, 04.02.2021 04:10

Physics, 04.02.2021 04:10


Mathematics, 04.02.2021 04:10

Mathematics, 04.02.2021 04:10


Mathematics, 04.02.2021 04:10

Mathematics, 04.02.2021 04:10

Mathematics, 04.02.2021 04:10


English, 04.02.2021 04:10






