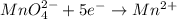Which of these half-reactions represents reduction?
I. Fe2+ → Fe3+
II. Cr2O72- → Cr3+
I...

Chemistry, 01.06.2021 21:50 maytce7237
Which of these half-reactions represents reduction?
I. Fe2+ → Fe3+
II. Cr2O72- → Cr3+
III. MnO4- → Mn2+

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Questions


Social Studies, 14.10.2019 20:30

History, 14.10.2019 20:30

History, 14.10.2019 20:30


Mathematics, 14.10.2019 20:30


English, 14.10.2019 20:30






Mathematics, 14.10.2019 20:30

Mathematics, 14.10.2019 20:30

English, 14.10.2019 20:30



Computers and Technology, 14.10.2019 20:30

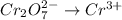
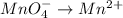
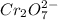 is +6 which is getting converted into +3, that is, decrease in oxidation state is taking place as follows.
is +6 which is getting converted into +3, that is, decrease in oxidation state is taking place as follows.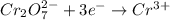
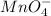 is +7 which is getting converted into +2, that is, decrease in oxidation state is taking place as follows.
is +7 which is getting converted into +2, that is, decrease in oxidation state is taking place as follows.