
Chemistry, 01.06.2021 20:00 twistedhyperboles
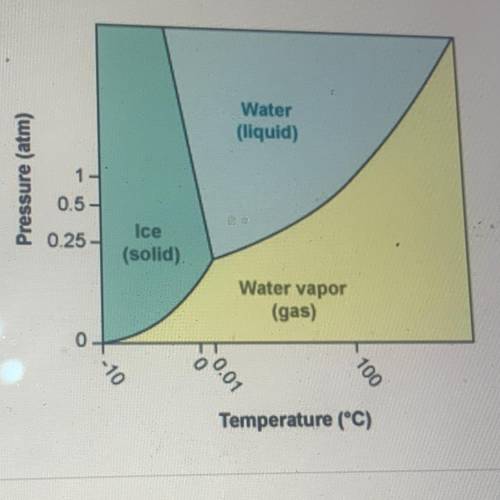
According to the phase diagram for H20, what happens to the phases of
water at 0°C as the pressure is increased from 0 atm to 10 atm?
A. Water changes from a gas to a solid to a liquid
B. Water changes from a liquid to a gas to a solid
C. Water changes from a liquid to a solid to a gas
D. Water changes from a solid to a liquid to a gas

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
According to the phase diagram for H20, what happens to the phases of
water at 0°C as the pressure...
Questions


Mathematics, 14.07.2021 16:10

English, 14.07.2021 16:10

Mathematics, 14.07.2021 16:10

Health, 14.07.2021 16:10


Mathematics, 14.07.2021 16:10

Mathematics, 14.07.2021 16:10

Mathematics, 14.07.2021 16:10





Mathematics, 14.07.2021 16:10


Mathematics, 14.07.2021 16:20



Mathematics, 14.07.2021 16:20

Mathematics, 14.07.2021 16:20



