
Chemistry, 31.05.2021 09:30 victoria6929
The gases in a hair spray can are at a temperature of 26.0 °C and a pressure of 25.0 lbs/in2. If the gases in the can reach a pressure of 90.0 Ibs/in?, the can will explode. To what temperature in Celsius must the gases be raised in order for the can to explode?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
The gases in a hair spray can are at a temperature of 26.0 °C and a pressure of 25.0 lbs/in2. If the...
Questions

Biology, 28.06.2019 14:40




Geography, 28.06.2019 14:40

Mathematics, 28.06.2019 14:40

English, 28.06.2019 14:40

Chemistry, 28.06.2019 14:40

Mathematics, 28.06.2019 14:40



History, 28.06.2019 14:40


Biology, 28.06.2019 14:40

History, 28.06.2019 14:40

History, 28.06.2019 14:40

Mathematics, 28.06.2019 14:40


Mathematics, 28.06.2019 14:40

Computers and Technology, 28.06.2019 14:40

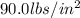 , what is the temperature of the gases?
, what is the temperature of the gases?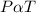
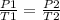
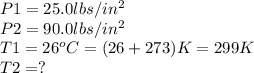

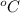 .
.

