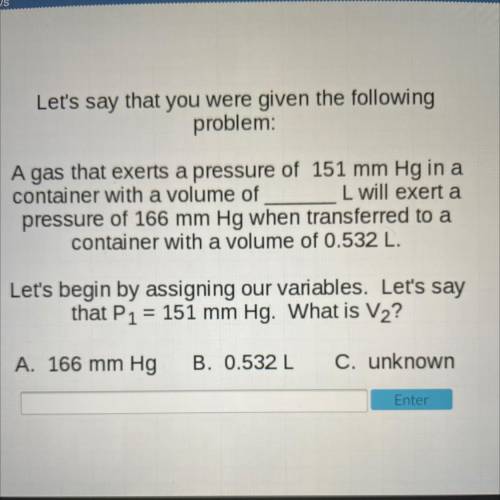
Let's say that you were given the following
problem:
A gas that exerts a pressure of 151 mm H...

Chemistry, 29.05.2021 21:00 moose12002
Let's say that you were given the following
problem:
A gas that exerts a pressure of 151 mm Hg in a
container with a volume of L will exert a
pressure of 166 mm Hg when transferred to a
container with a volume of 0.532 L.
Let's begin by assigning our variables. Let's say
that P1 = 151 mm Hg. What is V2?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Questions


Mathematics, 25.11.2020 02:10


Mathematics, 25.11.2020 02:10

Mathematics, 25.11.2020 02:10




History, 25.11.2020 02:10

History, 25.11.2020 02:10

Mathematics, 25.11.2020 02:10

English, 25.11.2020 02:10

English, 25.11.2020 02:10


Mathematics, 25.11.2020 02:10

Mathematics, 25.11.2020 02:10

English, 25.11.2020 02:10


Mathematics, 25.11.2020 02:10

English, 25.11.2020 02:10



