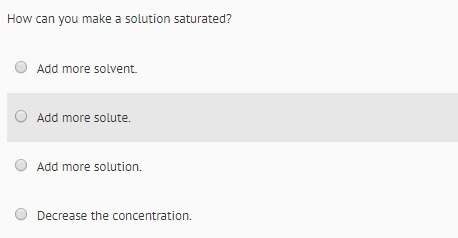
Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
You have 1.00 mol/L solution of sulfuric acid. Describe how you would use this solution to make 250....
Questions


Mathematics, 12.07.2019 09:50


Mathematics, 12.07.2019 09:50


Biology, 12.07.2019 09:50




Mathematics, 12.07.2019 09:50

Mathematics, 12.07.2019 09:50


English, 12.07.2019 09:50

Mathematics, 12.07.2019 09:50

Chemistry, 12.07.2019 09:50

World Languages, 12.07.2019 09:50


History, 12.07.2019 09:50