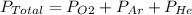Chemistry, 29.05.2021 04:30 bacchus2847
The total pressure of an O2-Ar-He gas mixture is 43 atm. If the partial pressure of Ar is 15 atm and the partial pressure of He is 7 atm, then the partial pressure of O2 is -

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2


Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
The total pressure of an O2-Ar-He gas mixture is 43 atm. If the partial pressure of Ar is 15 atm and...
Questions

Biology, 04.07.2019 21:30

Mathematics, 04.07.2019 21:30


History, 04.07.2019 21:30

Mathematics, 04.07.2019 21:30

Mathematics, 04.07.2019 21:30

Mathematics, 04.07.2019 21:30

Mathematics, 04.07.2019 21:30

History, 04.07.2019 21:30

Mathematics, 04.07.2019 21:30





History, 04.07.2019 21:30




Mathematics, 04.07.2019 21:30

Biology, 04.07.2019 21:30