
Chemistry, 28.05.2021 20:00 pedropaulofpedrosapp
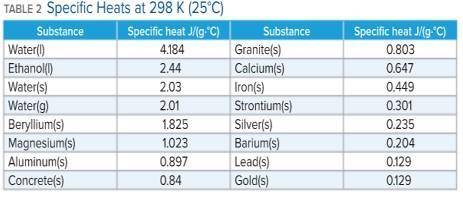
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substance absorbed 2440 J of energy. What is the specific heat of the substance? Identify the substance among those listed in Table 2.
A. the specific heat is 0.897 J/g. C, The Substance is aluminum
B. the specific heat is -0.897 J/g. C, The Substance is aluminum
C. the specific heat is 4.184 J/g. C, The Substance is water
D. there's not enough information to determine which is the substance.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

You know the right answer?
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substa...
Questions

English, 16.12.2020 14:00


Mathematics, 16.12.2020 14:00




Social Studies, 16.12.2020 14:00

Chemistry, 16.12.2020 14:00

English, 16.12.2020 14:00


Mathematics, 16.12.2020 14:00


Mathematics, 16.12.2020 14:00

Geography, 16.12.2020 14:00


Computers and Technology, 16.12.2020 14:00



Mathematics, 16.12.2020 14:00



