
Chemistry, 28.05.2021 17:40 bekahmc1p6k6vj
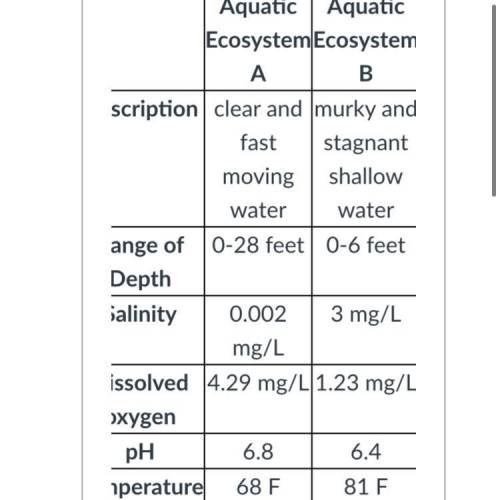
A scientist was studying two different aquatic ecosystems located in Northern Florida. The ecosystems are detailed in the table below.
Aquatic Ecosystem A
Aquatic Ecosystem B
Description
clear and fast moving water
murky and stagnant shallow water
Range of Depth
0-28 feet
0-6 feet
Salinity
0.002 mg/L
3 mg/L
Dissolved oxygen
4.29 mg/L
1.23 mg/L
pH
6.8
6.4
Temperature
68 F
81 F
Using the information from the table above, which aquatic ecosystem probably consists of more organisms?
Group of answer choices
Aquatic ecosystem B can support more life because it has a higher salinity than aquatic ecosystem B.
Aquatic ecosystem B can support more life because it is warmer and has shallower water.
Aquatic ecosystem A can support more life because it has more oxygen and circulating water.
Aquatic ecosystem A can support more life because it is deeper than aquatic ecosystem B.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
A scientist was studying two different aquatic ecosystems located in Northern Florida. The ecosystem...
Questions





Physics, 29.04.2021 05:10




Social Studies, 29.04.2021 05:10

Biology, 29.04.2021 05:10

Mathematics, 29.04.2021 05:10

Biology, 29.04.2021 05:10


Biology, 29.04.2021 05:10


Health, 29.04.2021 05:10







