
Chemistry, 28.05.2021 04:20 brillamontijo
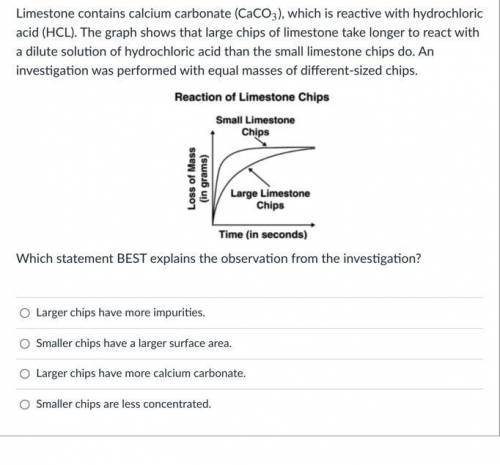
Limestone contains calcium carbonate (CaCO3), which is reactive with hydrochloric acid (HCL). The graph shows that large chips of limestone take longer to react with a dilute solution of hydrochloric acid than the small limestone chips do. An investigation was performed with equal masses of different-sized chips.
Which statement BEST explains the observation from the investigation?
Group of answer choices
Larger chips have more impurities.
Smaller chips have a larger surface area.
Larger chips have more calcium carbonate.
Smaller chips are less concentrated.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Limestone contains calcium carbonate (CaCO3), which is reactive with hydrochloric acid (HCL). The gr...
Questions


History, 28.01.2020 19:07

Mathematics, 28.01.2020 19:07


Biology, 28.01.2020 19:07

English, 28.01.2020 19:07


Social Studies, 28.01.2020 19:07

History, 28.01.2020 19:07


Health, 28.01.2020 19:07

Social Studies, 28.01.2020 19:07



History, 28.01.2020 19:07


Mathematics, 28.01.2020 19:07

English, 28.01.2020 19:07


Mathematics, 28.01.2020 19:07



