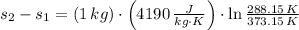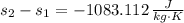Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
One kilogram of water at 100 0C is cooled reversibly to 15 0C. Compute the change in entropy. Specif...
Questions

Mathematics, 31.05.2021 08:20

Mathematics, 31.05.2021 08:20

English, 31.05.2021 08:20


English, 31.05.2021 08:20


Health, 31.05.2021 08:30

Physics, 31.05.2021 08:30


History, 31.05.2021 08:30

Chemistry, 31.05.2021 08:30




Health, 31.05.2021 08:30

Mathematics, 31.05.2021 08:30

Biology, 31.05.2021 08:30



 ), in joules per gram-Kelvin, by the following model:
), in joules per gram-Kelvin, by the following model: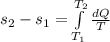
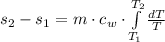
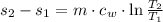 (1)
(1)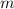 - Mass, in kilograms.
- Mass, in kilograms.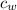 - Specific heat of water, in joules per kilogram-Kelvin.
- Specific heat of water, in joules per kilogram-Kelvin.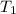 ,
, 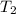 - Initial and final temperatures of water, in Kelvin.
- Initial and final temperatures of water, in Kelvin. 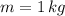 ,
, 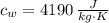 ,
, 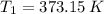 and
and 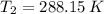 , then the change in entropy for the entire process is:
, then the change in entropy for the entire process is: