
Chemistry, 28.05.2021 01:10 aeshaalhemri
Some chemicals are acids and some are bases. We can test these chemicals with different indicators and determine if they are an acid or a base. Cabbage juice is an example of an acid-base indicator. If a solution of lemon juice is poured into purple cabbage juice, it will turn red. This indicates that it is an acid. If drain cleaner is added to cabbage juice, it will turn green indicating that it is a base.
Other materials can also be used as indicators if they change color in the presence of an acid or base. Litmus paper and phenolphthalein are commonly used in a chemistry lab but many other plant materials such as beets, blackberries and blueberries will also work.
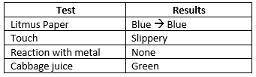
A student was given an unknown liquid. She tested it and recorded the following observations
Based on the information above, what was the most likely pH of the resulting solution?
3
5
7
12

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2

Chemistry, 23.06.2019 14:30
The first supersonic flight was in 1947. it was just above the speed of sound. which altitude would you expect captain yeager to have used for his flight
Answers: 3

Chemistry, 23.06.2019 16:00
Challenge question: this question is worth 6 points. as you saw in problem 9 we can have species bound to a central metal ion. these species are called ligands. in the past we have assumed all the d orbitals in some species are degenerate; however, they often are not. sometimes the ligands bound to a central metal cation can split the d orbitals. that is, some of the d orbitals will be at a lower energy state than others. ligands that have the ability to cause this splitting are called strong field ligands, cnâ’ is an example of these. if this splitting in the d orbitals is great enough electrons will fill low lying orbitals, pairing with other electrons in a given orbital, before filling higher energy orbitals. in question 7 we had fe2+, furthermore we found that there were a certain number (non-zero) of unpaired electrons. consider now fe(cn)6 4â’: here we also have fe2+, but in this case all the electrons are paired, yielding a diamagnetic species. how can you explain this?
Answers: 2
You know the right answer?
Some chemicals are acids and some are bases. We can test these chemicals with different indicators a...
Questions


Mathematics, 10.11.2020 21:00

English, 10.11.2020 21:00

English, 10.11.2020 21:00


Mathematics, 10.11.2020 21:00


Chemistry, 10.11.2020 21:00

English, 10.11.2020 21:00

Computers and Technology, 10.11.2020 21:00



Mathematics, 10.11.2020 21:00

History, 10.11.2020 21:00


History, 10.11.2020 21:00



Mathematics, 10.11.2020 21:00




