How much energy is released when 6.0 g of water is condensed from water
vapor?
A. 6.0 g x 1 m...

Chemistry, 28.05.2021 01:00 malachitorres813
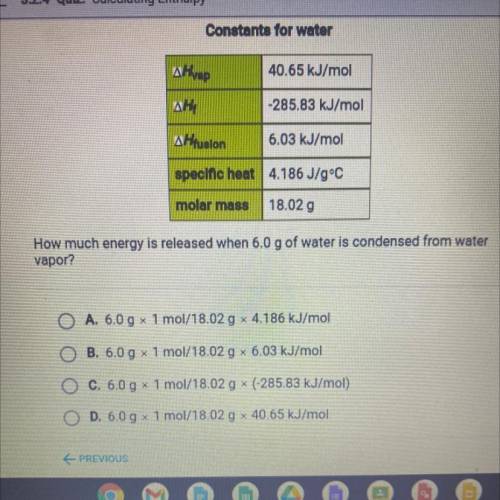
How much energy is released when 6.0 g of water is condensed from water
vapor?
A. 6.0 g x 1 mol/18.02 g x 4.186 kJ/mol
B. 6.0 g 1 mol/18.02 g 6.03 kJ/mol
O C. 6.0 g x 1 mol/18.02 g * (-285.83 kJ/mol)
O D. 6.0 g x 1 mol/18.02 g x 40.65 kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1


Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Questions

Biology, 18.11.2019 05:31

History, 18.11.2019 05:31



Physics, 18.11.2019 05:31

History, 18.11.2019 05:31


Social Studies, 18.11.2019 05:31

History, 18.11.2019 05:31

English, 18.11.2019 05:31


Chemistry, 18.11.2019 05:31

Mathematics, 18.11.2019 05:31







History, 18.11.2019 05:31



