Chemistry, 27.05.2021 22:40 microwave13016
1. For each of the following formulas:
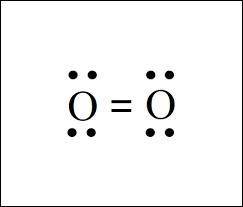
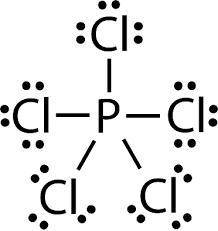
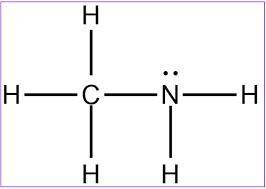
1) if ionic, write the formulas of the ions; if covalent, draw the Lewis structure
2) For each covalent compound, describe the electronic and molecular geometry
3) For each covalent compound, describe the hybridization of the central atom
4) Name each compound, except the organic one.
5) How many sigma and how many pi bonds does each compound have?
MnSO4 CH3NH2 PCl5 O2 LiF


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1


Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
1. For each of the following formulas:
1) if ionic, write the formulas of the ions; if covalent, dr...
Questions


Mathematics, 19.11.2020 22:10

Mathematics, 19.11.2020 22:10

Biology, 19.11.2020 22:10




Mathematics, 19.11.2020 22:10

Mathematics, 19.11.2020 22:10

Health, 19.11.2020 22:10

Mathematics, 19.11.2020 22:10

English, 19.11.2020 22:10


Mathematics, 19.11.2020 22:10

History, 19.11.2020 22:10


Spanish, 19.11.2020 22:10