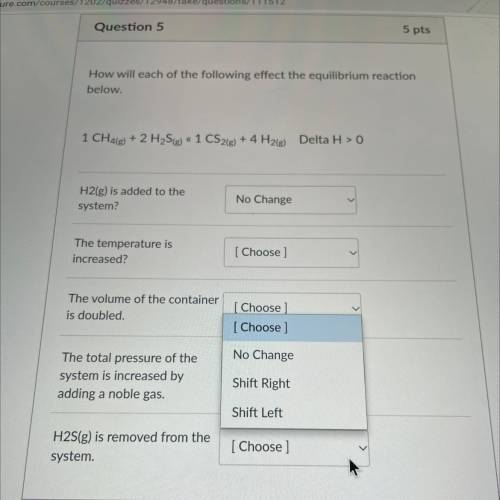
How will each of the following effect the equilibrium reaction
below.
1 CH4(g) + 2 H2S(g) « 1...

Chemistry, 27.05.2021 21:10 gerardoblk5931
How will each of the following effect the equilibrium reaction
below.
1 CH4(g) + 2 H2S(g) « 1 CS2(g) + 4 H2(g) Delta H > 0
HELPOPO

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2


Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
Questions

English, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00


Social Studies, 11.12.2020 01:00


Social Studies, 11.12.2020 01:00


English, 11.12.2020 01:00

History, 11.12.2020 01:00


History, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

History, 11.12.2020 01:00


English, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

Biology, 11.12.2020 01:00


Arts, 11.12.2020 01:00



