Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1


Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
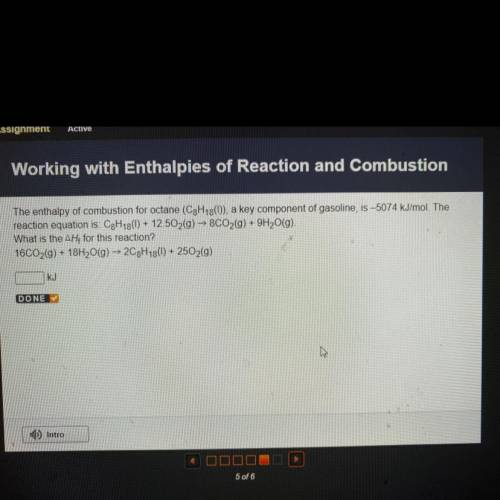
The enthalpy of combustion for octane (C3H13()), a key component of gasoline, is –5074 kJ/mol. The...
Questions

Mathematics, 01.09.2020 18:01

Social Studies, 01.09.2020 18:01

English, 01.09.2020 18:01

Mathematics, 01.09.2020 18:01


History, 01.09.2020 18:01





Mathematics, 01.09.2020 18:01

Chemistry, 01.09.2020 18:01

Mathematics, 01.09.2020 18:01



Computers and Technology, 01.09.2020 18:01


Chemistry, 01.09.2020 18:01

Computers and Technology, 01.09.2020 18:01

Mathematics, 01.09.2020 18:01