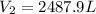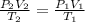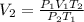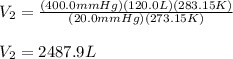Chemistry, 27.05.2021 06:30 qveenjordan6456
1. A volume of 120.0 liters of a gas is prepared at a pressure of 400.0 mm Hg and a temperature
100.0 °C. The gas is placed into a tank under high pressure. When the tank cools to 10.0°C,
the pressure of the gas is 20.0 mm Hg. What is the volume of the gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
1. A volume of 120.0 liters of a gas is prepared at a pressure of 400.0 mm Hg and a temperature
100...
Questions

Mathematics, 29.01.2020 12:46

Social Studies, 29.01.2020 12:46


Health, 29.01.2020 12:46



Mathematics, 29.01.2020 12:46



Mathematics, 29.01.2020 12:46

Mathematics, 29.01.2020 12:46


Mathematics, 29.01.2020 12:47







Mathematics, 29.01.2020 12:47