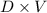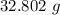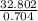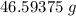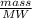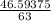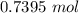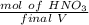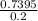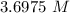Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3


Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
A solution is made by adding 23.1 mL of concentrated nitric acid ( 70.4 wt% , density 1.42 g/mL ) to...
Questions







Mathematics, 07.05.2020 01:00

Mathematics, 07.05.2020 01:00



Spanish, 07.05.2020 01:00

Mathematics, 07.05.2020 01:00

English, 07.05.2020 01:00


Mathematics, 07.05.2020 01:00

Social Studies, 07.05.2020 01:00

Chemistry, 07.05.2020 01:00