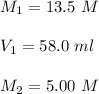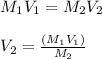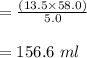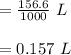Chemistry, 26.05.2021 20:50 dayanirisr45
A chemist must dilute of aqueous potassium iodide solution until the concentration falls to . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

You know the right answer?
A chemist must dilute of aqueous potassium iodide solution until the concentration falls to . He'll...
Questions

Mathematics, 30.03.2020 22:38

Mathematics, 30.03.2020 22:38

Arts, 30.03.2020 22:38

Mathematics, 30.03.2020 22:38










History, 30.03.2020 22:38

Mathematics, 30.03.2020 22:38


Mathematics, 30.03.2020 22:38

History, 30.03.2020 22:38


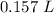 "
"