
Chemistry, 26.05.2021 19:00 kamster911
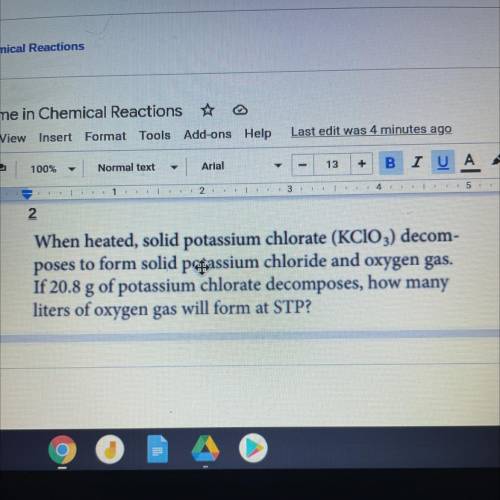
When heated, solid potassium chlorate (KClO3) decom-
poses to form solid passium chloride and oxygen gas.
If 20.8 g of potassium chlorate decomposes, how many
liters of oxygen gas will form at STP?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1


Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
When heated, solid potassium chlorate (KClO3) decom-
poses to form solid passium chloride and oxyge...
Questions

Mathematics, 31.07.2019 04:30

Biology, 31.07.2019 04:30

History, 31.07.2019 04:30


English, 31.07.2019 04:30

Biology, 31.07.2019 04:30

Physics, 31.07.2019 04:30






Computers and Technology, 31.07.2019 04:30

Biology, 31.07.2019 04:30








