
Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 13:20
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2

Chemistry, 23.06.2019 18:00
Amolecule is a(n) - a. element that physically combines with another element. b. particle composed of two or more atoms bonded together covalently. c. element that isn't bonded to another element.
Answers: 1
You know the right answer?
Calculate the pH of 1.0 X 10^-4 M HNO3...
Questions

History, 19.05.2021 21:20

Chemistry, 19.05.2021 21:20

Mathematics, 19.05.2021 21:20

History, 19.05.2021 21:20


Engineering, 19.05.2021 21:20


History, 19.05.2021 21:20

Mathematics, 19.05.2021 21:20


Spanish, 19.05.2021 21:20


Mathematics, 19.05.2021 21:20

Mathematics, 19.05.2021 21:20

Mathematics, 19.05.2021 21:20





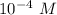
![pH=-log[H^+]](/tpl/images/1348/5259/15713.png)
![pH=-log[1\times 10^{-4}]\\\\pH=4](/tpl/images/1348/5259/8f6b7.png)


